Biosera Inc. ventured the distribution of vaccines brands from Serum Institute of India Pvt. Ltd., the worlds largest WHO-prequalified vaccine manufacturer by number of doses produced and sold globally.
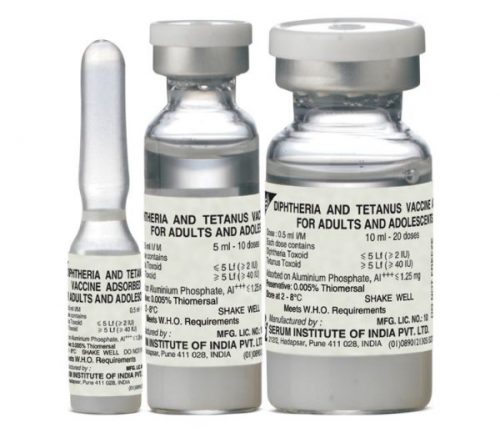
Td Vaccine
Tetanus and Diphtheria (Td) Vaccine for Adults and Adolescents
-
INDICATION
Indicated for primary vaccination and revaccination of adults and adolescents having contraindications of Pertussis antigen. Primary vaccination and revaccination of children older than 7 years.
-
ROUTE OF ADMINISTRATION
Intramuscular.
-
DOSAGE
Two injections of 0.5 ml. at least four weeks apart followed by a third injection 6 to 12 months after the second dose. The vaccine should also be given as a booster immunisation every 5 to 10 years.
-
COMPOSITION
Each single 0.5 ml human dose contains
Diphtheria Toxoid ≤ 5 Lf (≥ 2 IU)
Tetanus Toxoid ≥ 5 Lf (≥40 IU)
Adsorbed on Aluminium Phosphate, Al+++ ≤ 1.25 mg
Preservative: 0.005% Thiomersal
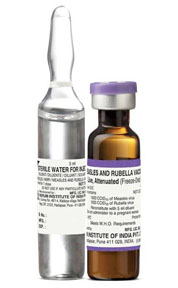
MR Vaccine
Measles and Rubella Vaccine
-
INDICATION
For active immunization against Measles and Rubella in infants, children, Adolescents and young adults at risk.
-
ROUTE OF ADMINISTRATION
Subcutaneous injection
-
DOSAGE
In Infants, children above 10 years, adolescents and adults, MR vaccine is recommended If pregnancy is planned then an interval of 1 month should be observed after MR vaccination.
-
COMPOSITION
Each single human dose when reconstituted in a volume of 0.5ml contains not less than 1000 CCID50 of live Measles virus particles and 1000 CCID50 of Rubella virus.
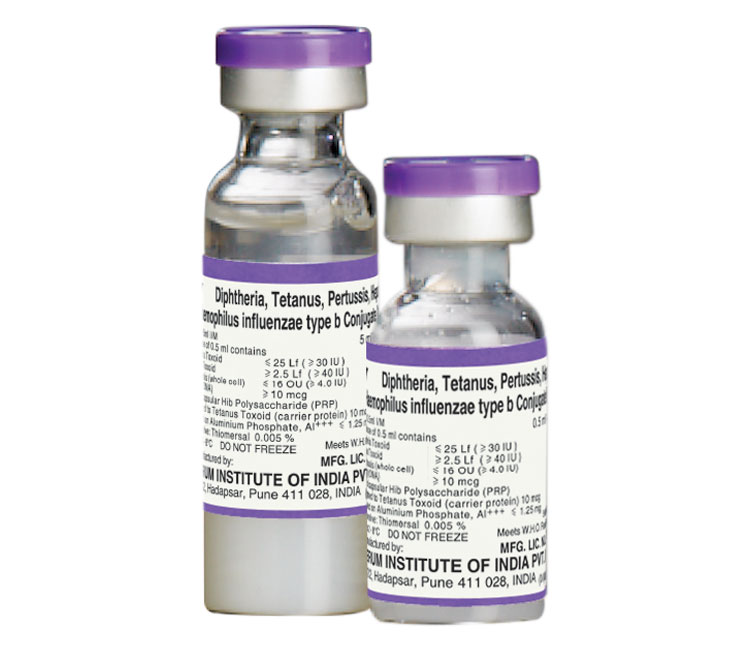
Pentavalent (5in1) Vaccine
DwPT-HepB-HiB Conjugate Vaccine Adsorbed
-
INDICATION
For the prevention of serious childhood diseases: Diphtheria, Tetanus, Pertussis (whooping cough), Hepatitis B and Haemophilus Influenzae type b
-
ROUTE OF ADMINISTRATION
Intramuscular.
-
DOSAGE
For active immunization of infants and pre-school children
It is recommended that three intramuscular injection of 0.5 ml be administered with an interval of four weeks between doses.
The first dose is recommended to be given at 6 weeks of age. -
COMPOSITION
Each dose of 0.5 ml contains
Diphtheria Toxoid ≤ 25 Lf (≥ 30 IU)
Tetanus Toxoid ≥ 2.5 Lf (≥ 40 IU)
B. pertussis (whole cell) ≤ 16 OU (≥ 4.0 IU)*
HBsAg (rDNA) ≥ 10 mcg
Purified capsular Hib Polysaccharide (PRP)
Conjugated to Tetanus Toxoid (carrier protein)10 mcg +++
Adsorbed on Aluminium Phosphate, Al ≤ 1.25 mg
Preservative: Thiomersal 0.005%
*The lower fiducial limit (P=0.95) of the estimated potency is not less than 2.0 IU. Adsorbed can be given at the age of 15-18 months.
